Single-cell RNA sequencing of pediatric Hodgkin lymphoma to study the inhibition of T cell subtypes
Jurrian K de Kanter, Alexander S Steemers, Diego Montiel Gonzalez, Ravian L van Ineveld, Catharina Blijleven, Niels Groenen, Laurianne Trabut, Marijn A Scheijde-Vermeulen, Liset Westera, Auke Beishuizen, Anne C Rios, Frank C P Holstege, Arianne M Brandsma, Thanasis Margaritis, Ruben van Boxtel, Friederike Meyer-Wentrup
Hemashpere. 2024 Sep 2;8(9):e149. doi: 10.1002/hem3.149
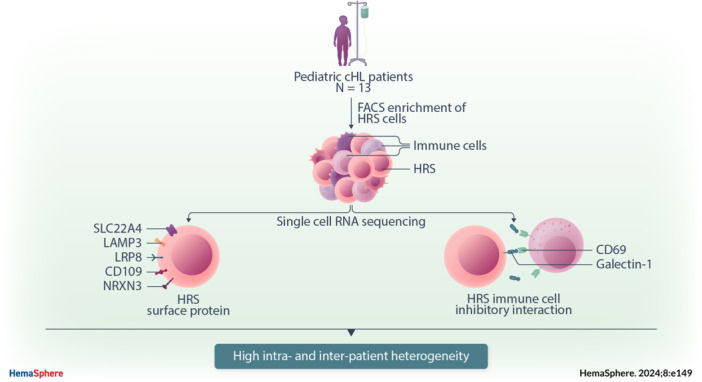
Pediatric classic Hodgkin lymphoma (cHL) patients have a high survival rate but suffer from severe long‐term side effects induced by chemo‐ and radiotherapy. cHL tumors are characterized by the low fraction (0.1%–10%) of malignant Hodgkin and Reed–Sternberg (HRS) cells in the tumor. The HRS cells depend on the surrounding immune cells for survival and growth. This dependence is leveraged by current treatments that target the PD‐1/PD‐L1 axis in cHL tumors. The development of more targeted therapies that are specific for the tumor and are therefore less toxic for healthy tissue compared with conventional chemotherapy could improve the quality of life of pediatric cHL survivors. Here, we applied single‐cell RNA sequencing (scRNA‐seq) on isolated HRS cells and the immune cells from the same cHL tumors. Besides TNFRSF8 (CD30), we identified other genes of cell surface proteins that are consistently overexpressed in HRS cells, such as NRXN3 and LRP8, which can potentially be used as alternative targets for antibody–drug conjugates or CAR T cells. Finally, we identified potential interactions by which HRS cells inhibit T cells, among which are the galectin‐1/CD69 and HLA‐II/LAG3 interactions. RNAscope was used to validate the enrichment of CD69 and LAG3 expression on T cells near HRS cells and indicated large variability of the interaction strength with the corresponding ligands between patients and between tumor tissue regions. In conclusion, this study identifies new potential therapeutic targets for cHL and highlights the importance of studying heterogeneity when identifying therapy targets, specifically those that target tumor‐immune cell interactions.


